What is SimpleTrials?
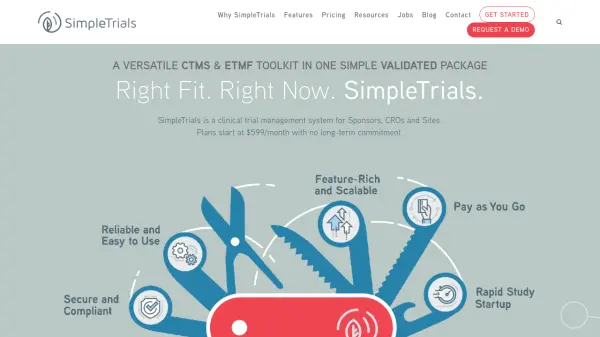
SimpleTrials is a validated clinical trial management system (CTMS) and eTMF solution designed for sponsors, clinical research organizations (CROs), and sites. It combines robust document management, subject tracking, site visit planning, automated workflows, and integrated analytics in a secure, compliant environment. Users benefit from flexible subscription plans with no multi-year contracts and scalable options tailored to team size and study complexity.
The platform supports seamless collaboration and oversight by offering integrated tracking for studies, documents, calendars, contracts, payments, and more. Features such as automated notifications, customizable trackers, risk management, and compliance documentation empower research teams to efficiently manage specialized studies or large portfolios while maintaining regulatory requirements and data integrity.
Features
- CTMS & eTMF Integration: unified management for studies, sites, and documents
- Compliance and Security: validated, 21 CFR Part 11 compliance, and dedicated compliance portal
- Custom Trackers: tailor data tracking for unique research needs
- Automated Alerts & Workflows: automated notifications via email and in-app for tasks and milestones
- Calendar & Site Visit Tracking: centralized scheduling and visit planning
- Subject and Enrollment Tracking: monitor screening, enrollment, protocol deviations and subject visits
- Contract and Payment Management: integrated solution for contracts, accrual reporting, and site/vendor payments
- Data Import & Integration: support for EDC/IRT integration and data workflow automations
- Advanced Reporting: real-time dashboards, ad-hoc report generation, analytics
- Premium Support: user manuals, videos, knowledge base, sandbox study, and help desk
Use Cases
- Managing and tracking clinical trials for pharmaceutical or biotech sponsors
- Electronic document management and regulatory compliance for clinical research organizations
- Coordinating site operations, subject enrollment, and visit scheduling in multi-site studies
- Automating workflows and notifications for study teams to ensure milestone completion
- Integrating with EDC, IRT, and other systems for streamlined data collection and reporting
FAQs
-
What compliance standards does SimpleTrials follow?
SimpleTrials is fully validated and 21 CFR Part 11 compliant, providing documentation and support to meet regulatory requirements. -
Can SimpleTrials be integrated with EDC or IRT systems?
Yes, SimpleTrials offers EDC/IRT integration as an add-on, enabling seamless data flow between systems. -
Is there a contract commitment required for subscriptions?
No, SimpleTrials offers monthly subscriptions with no multi-year contract required. -
Does SimpleTrials support document management for eTMF?
Yes, the platform supports electronic trial master file (eTMF) management with document archiving, QC workflow, and TMF downloads. -
What support resources are included with SimpleTrials subscriptions?
Subscribers have access to user manuals, video tutorials, a knowledge base, live help desk, and sandbox environments.
Related Queries
Helpful for people in the following professions
Featured Tools
Join Our Newsletter
Stay updated with the latest AI tools, news, and offers by subscribing to our weekly newsletter.